제조공정








강철 열처리의 야금학적 원리
강철의 열처리는 강철의 야금학적 구조 특성에서 원하는 변화를 달성하기 위해 수행됩니다. 열처리에 의해 강철은 특성에 심한 변화를 겪습니다. 일반적으로 강철이 고온 오스테나이트 상태로 가열된 다음 거의 평형 상태에서 천천히 냉각될 때 매우 안정적인 강철 구조가 얻어집니다. 일반적으로 어닐링 또는 노멀라이징으로 알려진 이러한 유형의 열처리는 강철 내에 고정된 낮은 수준의 잔류 응력을 갖는 구조를 생성하며 구조는 Fe(철)-C(탄소) 평형 다이어그램에서 예측할 수 있습니다. 그러나 강재에서 가장 많이 요구되는 특성은 높은 강도와 경도이며 일반적으로 높은 수준의 잔류응력을 동반합니다. 이는 비평형 냉각 또는 오스테나이트 상태의 담금질에 의해 생성된 준안정 구조 때문입니다.
결정 구조 및 상
고체 상태의 순수한 Fe의 결정 구조는 두 가지 동소 상태로 존재하는 것으로 알려져 있습니다. 주위 온도에서 최대 910℃까지 Fe는 체심 입방체(bcc) 격자를 가지며 알파-철(alpha-Fe)이라고 합니다. 섭씨 910도에서 알파-철(alpha-Fe) 결정은 면심 입방(fcc) 격자를 가진 감마-철 결정으로 변합니다. 감마 결정은 최대 1400℃의 온도까지 안정성을 유지합니다. 이 온도 이상에서 그들은 델타 결정으로 알려진 bcc 격자를 다시 얻습니다. 델타 결정은 존재하는 온도 영역에서만 알파 결정과 다릅니다. Fe에는 두 개의 격자 상수가 있습니다. 낮은 온도에서 alpha-Fe는 강한 강자성 특성을 보입니다. 이것은 격자가 강자성 스핀 순서를 잃기 때문에 약 770℃로 가열되면 사라집니다. 770℃ 이상의 Fe 상태를 베타-철(beta-Fe)이라고 합니다. 상자성 베타 결정의 격자는 알파 결정의 격자와 동일합니다.
한 형태에서 다른 형태로 진행되는 동안 Fe는 과냉각될 수 있습니다. 이로 인해 가열 및 냉각 시 변태점 위치의 차이가 발생합니다. 그 차이는 냉각 속도에 따라 달라지며 히스테리시스라고 합니다. 문자 'c'와 'r'은 변형이 가열 때문인지 냉각 때문인지 나타냅니다. 또한, alpha-Fe가 감마-Fe로 변환될 때 밀도의 변화는 물질의 부피에 급격한 변화를 초래합니다. 때로는 탄성 한계를 초과하여 실패로 이어지는 응력을 발생시킵니다. 감마-철의 밀도는 알파-철의 밀도보다 약 4% 높습니다.
철-탄소 평형 도표
Fe-C 합금인 강의 구조는 C가 풍부한 성분으로 순수한 C(흑연) 또는 시멘타이트(Fe3C)로 알려진 화합물을 포함할 수 있습니다. 시멘타이트는 비교적 천천히 냉각된 강철에도 존재합니다(Fe3C를 Fe 및 C로 분해하려면 일반적으로 더 높은 온도에서 오래 유지해야 함). 이러한 이유로 Fe-C 평형 다이어그램은 종종 Fe-Fe3C 평형 다이어그램으로 취급됩니다. Fe-C 다이어그램은 안정적이고 Fe-Fe3C 다이어그램은 준안정적입니다. Fe-C 안정 도표와 Fe-Fe3C 준안정 도표를 모두 포함하는 Fe-C 평형 도표는 그림 1에 나와 있습니다. 점선은 안정 Fe-C 도표를 나타내고 실선은 준안정 Fe-Fe3C 도표를 나타냅니다.
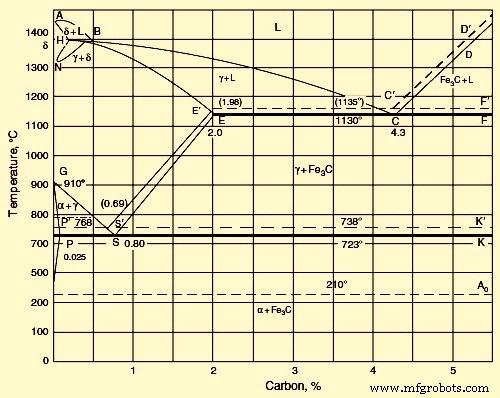
그림 1 철 탄소 도표
준안정 Fe-Fe3C 다이어그램에서 Fe(델타, 감마, 알파)의 동소체 형태의 격자는 델타, 감마 및 Fe에서 C의 고용체 형성 사이트 역할을 합니다. C가 결핍된 강이 결정화되면 델타 고용체의 결정이 액상선 AB와 고상선 AH에서 석출됩니다. 델타 고용체에는 bcc 격자가 있습니다. 1490℃의 최대 온도에서 델타 용액은 0.1% C(점 H)를 포함합니다. 1490℃에서 포화 델타 용액과 0.5% C를 함유한 액체 사이에 포정 반응이 일어납니다(B 지점). 그 결과, 감마 Fe에 C의 감마 고용체가 형성된다. 0.18% C(포인트 I)가 포함되어 있습니다.
C 함량이 0.5%보다 높으면 감마 고용체가 액체에서 직접 결정화됩니다(액상 BC 및 고체 IE에서). 1130℃에서 감마 Fe에서 C의 한계 용해도는 2.0%에 가깝습니다(E 지점). 1130℃에서 온도를 낮추면 라인 ES에서 감마-Fe의 C 용해도가 낮아집니다. 723℃에서 C의 용해도는 0.8%(S 지점)입니다. 선 ES는 감마 용액에서 Fe3C의 침전에 해당합니다.
C 함량이 증가할수록 감마 격자가 알파 격자로 변환되는 온도가 낮아지고, 곡선 GS 및 GP에 해당하는 온도 구간에 걸쳐 변환이 일어난다. 알파상 침전 곡선 GS는 Fe3C 침전 곡선 ES와 교차한다. 점 S는 좌표가 723°C 및 0.80%C인 공석점입니다. 이 지점에서 포화 알파 용액과 Fe3C 침전물이 동시에 공석 농도 감마 용액을 형성합니다. 알파 고용체의 격자는 델타 고용체의 격자와 동일합니다. 723℃의 공석 온도에서 알파 고용체는 0.02% C(포인트 P)를 포함합니다.
추가 냉각은 alpha-Fe에서 C 용해도를 낮추고 실온에서 이것은 퍼센트의 작은 부분과 같습니다(점 D). C 함량이 2% – 4.3%일 때 라인 BC에서 감마 용액의 침전으로 결정화가 시작됩니다. C 함량이 4.3% 이상으로 증가하면 라인 CD에서 Fe3C가 침전됩니다. 2.0% 이상의 C를 함유하는 모든 철 합금에서 잉여 1차상의 침전은 좌표가 1130℃ 및 4.3%C인 점 C에서 감마 용액 및 Fe3C의 공융 결정화로 이어집니다. 선 Ao는 자기 강자성에서 상자성 상태로의 전환인 변환입니다.
안정적인 Fe-C 평형 다이어그램의 경우 냉각 속도가 매우 낮기 때문에 C(흑연)가 액체에서 직접 결정화될 수 있습니다. 이 경우, 오스테나이트와 시멘타이트의 공정 대신에 오스테나이트와 흑연의 공정 혼합물이 형성됩니다. 그림 1에서 점선은 Fe-흑연 시스템을 상징합니다. 이 라인은 Fe-Fe3C 시스템의 라인보다 더 높은 온도에 있습니다. 이것은 Fe-흑연 시스템의 완전한 평형에 대한 더 큰 안정성과 근접성을 확인합니다. 이것은 또한 Fe3C가 많은 고 C 강의 가열이 Fe3C =3Fe + C 방정식으로 표시된 분해로 이어진다는 사실에 의해 뒷받침됩니다.
중간 냉각 속도에서 강의 일부는 흑연 시스템에 따라 결정화되고 다른 부분은 시멘타이트 시스템에 따라 결정화될 수 있습니다. 두 시스템의 다이어그램에서 상 평형선은 특정 냉각 속도에 따라 변위될 수 있습니다. 감마-철(오스테나이트)에서 C 고용체의 석출 선에 대해 현저한 변위를 볼 수 있습니다. 이러한 이유로 다이어그램은 상대적으로 느린 냉각 속도에 노출된 강철에 대해서만 완전히 사실입니다.
탄소의 영향
α-Fe에서 C의 최대 용해도는 721℃에서 관찰되며 0.018% C와 같습니다. 급냉에 따라 C는 알파 고용체에 남아 있을 수 있지만 곧 노화 메커니즘에 의해 상의 침전이 시작됩니다. 고용체에서 C는 (i) 균질한 용액, 드문 경우인 정적으로 균일한 간극 분포 또는 (ii) 불균일한 용액을 형성할 수 있습니다. 결정 격자 구조가 교란되는 곳(입계, 전위)에서 클러스터 형성. 후자는 가장 가능성이 높은 고용체 상태입니다. 이렇게 형성된 클러스터는 소성 변형 중 전위 이동에 대한 장애물을 나타내며 소성 흐름의 시작 부분에서 변형의 불균일한 발달을 담당합니다.
Fe – C 합금에 대한 C 함량의 영향을 분석하려면 모든 구조적 구성요소를 고려해야 합니다. 천천히 냉각된 강은 페라이트와 시멘타이트 또는 페라이트와 흑연으로 구성됩니다.
페라이트는 플라스틱입니다. 소둔 상태에서 페라이트는 연신율이 크고(약 40%), 연질(브리넬 경도는 결정 치수에 따라 65-130임)이며 최대 770℃까지 강자성입니다. 723℃에서 0.22% C가 용해됩니다. 페라이트이지만 실온에서는 용액에 C의 1/1000만 남습니다.
시멘타이트는 부서지기 쉽고 더 높은 경도를 나타냅니다(브리넬 경도는 약 800). 섭씨 210도까지 약하게 자성을 띠며 전기와 열을 잘 전도하지 못한다. 복잡한 마름모꼴 격자를 가지고 있습니다. 일반적으로 (i) 라인 CD에서 액체로부터 결정화되는 1차 Fe3C, (ii) 라인 ES에서 감마 용액으로부터 침전되는 2차 Fe3C, (iii) PQ 라인의 솔루션입니다.
흑연은 부드럽습니다. 전기 전도성이 좋지 않지만 열을 잘 전달합니다. 흑연은 3000~3500℃의 온도에서도 녹지 않습니다. 축 관계 c/a가 2보다 큰 육각 격자를 가지고 있습니다.
오스테나이트는 부드럽고(하지만 페라이트보다 단단함) 연성입니다. 오스테나이트의 연신율은 40%~50%입니다. 페라이트보다 열과 전기의 전도율이 낮고 상자성입니다. 오스테나이트는 fcc 격자를 가지고 있습니다.
0 % - 0.02 % C를 포함하는 강의 조직은 페라이트와 3차 Fe3C로 구성됩니다. C 함량의 추가 증가는 페라이트와 Fe3C(펄라이트)의 공석인 새로운 구조적 구성요소의 출현으로 이어집니다. 펄라이트는 먼저 페라이트 입자 사이에 별도의 개재물로 나타나고 0.8% C에서 전체 부피를 차지합니다. 펄라이트는 일반적으로 라멜라 구조를 갖는 2상 혼합물을 특징으로 합니다. 강의 C 함량이 0.8% 이상으로 증가함에 따라 펄라이트와 함께 2차 Fe3C가 형성된다. 2차 Fe3C는 바늘 모양입니다. Fe3C의 양은 C 함량이 증가함에 따라 증가한다. 2% C에서는 현미경 시야의 18%를 차지합니다. C 함량이 2%를 초과하면 공융 혼합물이 나타납니다. 급속 냉각된 강철에서 모든 잉여 상(페라이트 또는 Fe3C)이 공석이 형성되기 전에 침전할 시간이 있는 것은 아닙니다.
3.6% C의 합금에는 ledeburite(감마-Fe와 Fe3C의 C 고용체의 공융 혼합물)가 포함되어 있습니다. 합금은 저공정 백색 주철로 더 적절하게 분류됩니다.
임계(변환) 온도
탄소는 고체 상태에서 Fe의 변형에 현저한 영향을 미칩니다. Fe-C 평형 다이어그램에서 선 GS 및 NL의 위치 s는 C 함량의 증가가 그림 1에 표시된 대응물에 비해 점 A3의 낮추고 점 A4의 상승으로 이어진다는 것을 보여줍니다. 따라서 C는 확장 델타 상의 온도 범위.
공석(펄라이트)이 형성되면 가열 및 냉각 곡선이 정지됩니다. 이것은 점 A1(가열 시 Ac1, 냉각 시 Ar1)으로 표시됩니다. 이 현상은 0.9% C에서 발생합니다(Fe–C 다이어그램의 점 S). 차아공석강의 페라이트 침전(선 GOS 교차 시)은 가열 및 냉각 곡선에서 A3 지점으로 표시되는 변곡선으로 나타납니다. 이 점은 순철의 감마에서 알파로의 변환에 해당합니다. 공석 침전에 선행하는 Fe3C의 침전(선 ES의 교차)은 냉각 곡선에서 점 Acm(가열 시 Ac,cm 및 냉각 시 Ar,cm)로 지정된 약한 굴곡으로 나타납니다. C의 첨가는 자기 변태 온도(점 A2)에 거의 영향을 미치지 않습니다. 따라서 선 MO는 C 함량이 낮은 강의 자기 변형에 해당합니다. 더 많은 양의 C를 포함하는 합금에서 이러한 변형은 페라이트 석출의 시작에 해당하는 라인 GOS에서 발생합니다. C 함량이 점 S에 해당하는 것보다 높으면 자기 변형이 온도 A1과 일치합니다.
시멘타이트는 자기 변형을 겪습니다. C 함량이 무엇이든 간에 변형은 210°C–220°C의 온도에서 발생합니다. A2 지점에서 순수한 Fe의 자기 변형과 마찬가지로 현저한 히스테리시스 없이 발생합니다.
강철의 구조적 변형
강철을 경화시킬 때 고온으로 가열하여 전체 조직을 고온에서 안정한 Fe와 C의 단일상 조직인 오스테나이트상으로 변환합니다. 이 가열된 강을 천천히 냉각시키면 오스테나이트는 상온에서 평형상인 펄라이트로 변태한다. 펄라이트 조직은 어닐링된 조직으로 물성이 낮고 비교적 부드럽다. 가열된 강을 매우 빠르게 냉각하면 철에 용해된 C의 준안정상인 마르텐사이트라고 하는 단단하고 강한 조직이 형성됩니다. 이 상은 덜 취성인 더 낮은 경도 구조를 생성하도록 템퍼링될 수 있습니다. 중간 냉각 속도는 베이나이트와 같은 다른 구조를 생성하지만 이러한 유형의 구조는 합금강에서만 소량 생산됩니다. 공석 C강은 냉각 속도에 따라 주로 마르텐사이트 또는 펄라이트를 생성합니다.
오스테나이트 펄라이트 변태
오스테나이트의 fcc 격자에서 페라이트의 bcc 격자로의 변환은 오스테나이트에 용해된 C의 존재로 인해 방해를 받습니다. 오스테나이트 격자는 셀의 중심에 C 원자를 수용할 수 있는 충분한 공간을 가지고 있습니다. 페라이트의 bcc 격자에는 이 공간이 없습니다. 이로 인해 C의 용해도는 오스테나이트에서 페라이트로 전이할 때 상당히 낮아집니다. 베타에서 알파로의 변환 동안 거의 전체 C가 오스테나이트 격자에서 석출됩니다. 준안정 Fe-Fe3C 도표에 따르면 시멘타이트로 침전됩니다. 이 변환은 (i) gamma-Fe 격자에서 alpha-Fe 격자로의 변환, (ii) Fe3C로 C의 침전, (iii) 탄화물의 응고라는 세 가지 상호 연결된 경로로 정의할 수 있습니다.
점 A1의 온도에서 경로 (i) 및 (ii)에 의한 변태는 거의 동시에 진행되어 페라이트와 시멘타이트의 층상 혼합물이 형성됩니다. 용해된 C의 원자는 격자에 무작위로 분포되어 있습니다. 이로 인해 Fe3C는 C가 풍부한 영역에서 핵을 생성하고 탄소가 거의 없는 영역에서 페라이트를 생성합니다. 이러한 C의 재분배는 확산을 통해 이루어지며 온도와 시간에 따라 달라집니다.
0.8% 미만의 C를 함유하는 차공석강을 서냉하면 결정립계에서 페라이트가 형성되면서 변태가 시작된다. 이 입계는 페라이트 결정화 중심으로 작용합니다. 탄소는 결정자 내부에 강제로 들어가게 됩니다. 페라이트가 석출됨에 따라 페라이트 형성에 필요한 농도가 중앙 체적에서 달성됩니다. 초공석강(C 0.8% 이상)을 서냉하면 ES선을 넘을 때 Fe3C가 입계에서 석출되기 시작한다. 여기에서 결정립계는 결정화 사이트 역할도 합니다.
γ-Fe와 alpha-Fe 격자의 C 확산율은 온도가 낮아질수록 확산계수가 온도에 의존하기 때문에 급격히 감소한다. 적절한 냉각 속도를 제공하면 펄라이트 형성이 불가능할 정도로 과냉각이 향상될 수 있습니다.
저온 범위에서 변형 메커니즘과 형성된 구조의 특징은 변형이 일어나는 온도에만 의존합니다. 과냉도를 고려하여 (i) 펄라이트 범위, (ii) 중간 범위, (iii) 마르텐사이트 범위의 3가지 변태 온도 범위로 구분됩니다. 한 변환 메커니즘에서 다른 변환 메커니즘으로의 지속적인 전환은 이러한 온도 범위에서 발생할 수 있습니다. 변형 과정은 강철의 C 및 기타 원소의 함량에 크게 의존합니다. 더 빠른 메커니즘으로 시작하여 더 느린 메커니즘으로 끝날 수 있습니다.
펄라이트 범위에서 변태는 페라이트와 탄화물의 혼합물이 동시에 형성되는 것이 특징입니다. 자유 페라이트 또는 탄화물은 오스테나이트 입계에서 침전될 수 있습니다. 여기에서 두 단계의 형성과 성장은 확산 과정에 의해 제어됩니다. Fe 및 기타 요소의 확산이 중요한 역할을 합니다. 페라이트 및 탄화물의 확산 결정화에 더 오랜 시간이 필요할 때까지 온도가 낮아질수록 조직 미세도가 향상됩니다.
펄라이트는 펄라이트 범위에서 변형될 때 형성되는 페라이트와 카바이드 판의 기계적 혼합물입니다. 펄라이트 결정핵이 형성되는 속도는 오스테나이트와 탄화물의 과포화도에 따라 달라지는데, 이는 온도가 낮아질수록 증가한다. 속도는 또한 온도에 따라 감소하는 확산 속도에 따라 달라집니다. 펄라이트 섬의 성장은 주로 C 및 Fe 원자의 확산 속도에 따라 달라집니다. 다른 요인은 (i) 과포화 정도 및 (ii) 페라이트 형성 중 자유 에너지 이점입니다. 펄라이트 섬은 새로운 판의 형성뿐만 아니라 모든 방향에서 오래된 판의 추가 성장을 통해 성장합니다. 카바이드 플레이트는 페라이트 플레이트보다 빠르게 성장합니다.
펄라이트 형성 과정은 페라이트 핵의 형성으로 시작됩니다. 페라이트와 시멘타이트 판의 핵 생성과 두 상 판의 분기가 여러 번 번갈아 가며 평면 평행 및 부채꼴 모양의 펄라이트 판을 형성합니다. 펄라이트 핵은 결정립계, 불용성 탄화물 또는 비금속 개재물과 같은 결정 구조 결함이 있는 격자 영역에서 주로 나타납니다. 펄라이트의 매우 중요한 특징은 플레이트 간 간격입니다. 간격을 줄이면 강철의 강도 특성이 향상됩니다.
펄라이트 범위에서 Fe3C 및 페라이트 결정화 중심의 형성 속도는 온도가 낮아질수록 가속화됩니다. 구조의 미세도가 증가하면 플레이트 간 간격이 감소합니다.
강철의 특성에 영향을 미치는 중요한 특징은 펄라이트 군락의 치수입니다. 콜로니 치수의 감소는 충격 강도의 증가 및 취성의 감소를 동반합니다. 임계 취성 온도는 펄라이트 형태에 따라 다릅니다. 따라서 페라이트와 시멘타이트 판의 파단시 비교적 고강도의 펄라이트가 형성되어 페라이트 내부에 고밀도의 전위를 형성한다.
펄라이트의 더 나은 파괴 강도는 Fe3C 입자의 구상화를 통해 달성됩니다. 구상화는 펄라이트의 변형, 후속 가열 및 Ac1 근처의 온도에서 유지함으로써 촉진될 수 있습니다. 펄라이트의 상대적으로 높은 강도와 연성을 제공하는 또 다른 방법은 펄라이트 변태 동안의 변형으로 구성됩니다. 이것은 다각형 구조의 형성과 시멘타이트의 구상화로 이어진다. 페라이트-펄라이트 혼합물의 항복응력(YS)은 페라이트와 펄라이트의 특성에 따라 부가적인 방식으로 달라집니다.
오스테나이트의 변태
차공석강과 초공석강에서 오스테나이트가 변태하는 동안 펄라이트 변태는 페라이트와 2차 시멘타이트와 같은 과잉 상의 침전이 선행됩니다. 구조적으로 자유로운 과잉 상의 상대적인 양은 오스테나이트 과냉각 정도에 따라 달라집니다. 과잉 페라이트 또는 Fe3C의 양은 냉각 속도가 증가함에 따라 감소합니다. 적절한 정도의 과냉각이 있을 때 독립적인 구조적 구성요소로서 과잉 상의 형성을 피할 수 있습니다.
소량의 공석 오스테나이트를 함유하는 차공석강을 서냉에 노출시키면 과잉의 페라이트 결정립에 공석 페라이트가 성장하고, 결정립계에 구조적으로 자유로운 층간 Fe3C가 남게 된다. 초 공석 강철에서 공석은 또한 구조적 변질의 대상이 될 수 있습니다. A1 지점(700℃ 이상) 이하의 매우 낮은 냉각 하에서 공석 석출로 인해 형성된 시멘타이트는 2차 시멘타이트에 퇴적된다. 구조적으로 자유로운 페라이트 영역이 옆에 나타납니다. 상 분리를 동반하는 이 공석 변형은 비정상으로 간주됩니다. 정상적인 공석 변형에서 페라이트와 Fe3C는 두 단계의 규칙적인 교대로 집락 형태로 함께 성장합니다. 비정상적인 변형의 경우 페라이트와 Fe3C의 조대한 혼합물은 독특한 공석 구조를 갖지 않습니다. 공석 변형 동안 메커니즘이 비정상에서 정상으로 변경될 수 있습니다. 따라서 급속 냉각과 그에 상응하는 오스테나이트의 현저한 과냉각으로 비정상적인 변태를 완전히 억제할 수 있습니다.
차공석강에서 과도한 페라이트의 경우, 페라이트는 (i) 조밀한 등축 결정립 및 (ii) 배향된 Widmanstätten 판의 두 가지 형태로 발견됩니다. 차공석 페라이트의 조밀한 석출물은 오스테나이트 결정립계에서 크게 나타나는 반면, Widmanstätten 판은 결정립 내부에 형성됩니다. Widmanstätten 페라이트는 0.4% 미만의 C 및 다소 거친 오스테나이트 입자를 갖는 강에서만 관찰됩니다. 오스테나이트 결정립의 치수가 감소함에 따라 등축 결정립 형태의 페라이트 비율이 증가합니다. Widmanstätten 페라이트는 A3(50 deg C)에서 600 deg C, 550 deg C의 온도 구간에 걸쳐 형성됩니다. 강철의 C 함량이 증가함에 따라 구조에서 Widmanstätten 페라이트의 비율이 낮아집니다.
Widmanstätten 페라이트는 정렬된 원자의 상호 관련된 운동을 수반하는 격자의 전단 감마-알파 재배열로 인해 형성되는 것으로 추정됩니다. 페라이트의 등축 입자는 감마/알파 경계를 가로질러 원자의 무질서한 전이와 함께 격자의 정상적인 확산 재배열에 의해 성장합니다.
강을 강화하는 데 사용되는 방법 중 하나는 분산된 탄화물 침전물을 포함하는 차공석 페라이트로 구조를 제공하는 것입니다. 이러한 구조를 생성하기 위해 강철은 특수 탄화물이 오스테나이트에 용해될 때까지 가열된 다음 차아 공석 페라이트가 형성되기 시작하기 전에 오스테나이트에서 직접 탄화물의 일반적인 석출을 방지하기 위해 빠르게 냉각되어야 합니다.
마르텐사이트의 변태
마르텐사이트의 변태는 고온 상의 담금질(급냉) 때문입니다. C 강의 마르텐사이트 변태의 주요 특성은 다음과 같습니다.
Ms-온도는 특정 전처리를 거친 특정 조성의 강철을 특징으로 합니다. 주어진 강철에서 마르텐사이트 변태는 냉각 속도에 관계없이 동일한 온도에서 시작됩니다. 이 온도는 강의 조성에 따라 달라지며 강의 C 함량이 증가함에 따라 크게 감소합니다. C의 일부는 오스테나이트와 공존하는 탄화물에 들어갑니다. 담금질 온도가 증가하면 탄화물이 오스테나이트에 용해됩니다. 따라서 오스테나이트의 C 농도는 증가하고 Ms point는 낮아진다.
마르텐사이트 형성은 오스테나이트 격자 재배열의 전단 메커니즘으로 간주된다. 상 변형의 마르텐사이트(전단) 메커니즘은 원자 사이의 간격보다 짧은 거리로 원자의 정렬된 상호 관련된 이동에 의해 잘 알려져 있으며 원자는 위치를 교환하지 않습니다. 초기 단계의 원자는 마르텐사이트 단계의 이웃을 보존합니다. 이것은 격자의 전단 재배열에 고유한 주요 기능입니다.
격자 재배열의 이러한 특성은 이전 단계와 새 단계 간의 경계 일관성을 제공합니다. 마르텐사이트와 초기 상 사이의 경계에서 격자의 일관성(탄성 접합)은 낮은 온도에서도 매트릭스를 향한 경계의 매우 빠른 이동을 보장합니다. 원자는 원자간 간격보다 짧은 거리로 협력하여 이동하여 마르텐사이트 결정의 성장을 초래합니다.
마르텐사이트 결정의 성장과 함께 탄성 변형률이 간섭 경계에 축적됩니다. YS에 도달하면 일관성이 방해받습니다. 원자는 마르텐사이트 결정과 시작 매트릭스 사이의 경계에서 무질서해집니다. 경계의 미끄러짐 움직임이 불가능하게 됩니다. 따라서, 마르텐사이트 메커니즘에 의한 결정의 성장은 종료되고, 이후 결정은 확산에 의해서만 성장할 수 있다. 그러나 마르텐사이트 변태는 확산 속도가 매우 작은 저온에서 발생합니다. 따라서 결맞음이 끊어진 후에는 마르텐사이트 결정의 성장이 거의 관찰되지 않습니다.
마르텐사이트 메커니즘에 의한 고용체의 다형 변형은 구성 요소의 확산 재분배가 없다는 특징이 있습니다. 고온 상이 저온 상으로 변태하는 마르텐사이트 메커니즘에 필요한 조건이 여기에 설명되어 있습니다. 작은 과냉각에서는 마르텐사이트 변태가 불가능합니다. 이것은 격자의 무질서한 재배열의 경우 탄성 변형이 부피의 변화에 의해서만 결정되는 반면, 마르텐사이트 변형의 경우 추가로 초기 및 마르텐사이트 결정의 격자의 일관성에 의존하기 때문입니다. 과냉각도가 높을수록 격자의 무질서한 재배열률이 증가하여 최대에 도달한 다음 감소합니다. Fe에서 다형 변태의 마르텐사이트 메커니즘을 얻으려면 강철을 감마 범위에서 강하게 과열한 다음 매우 빠르게 냉각하여 정상 변태의 진행을 억제해야 합니다.
마르텐사이트 형성 동안, 오스테나이트의 fcc 격자가 마르텐사이트의 bcc 정방 격자로 재배열되는데, 이는 알파-Fe의 bcc 격자와 유사합니다. 오스테나이트 격자는 c-축을 따라 오스테나이트의 정방 셀의 압축과 a-축을 따라 치수의 동시 증가로 구성된 베인 변형을 통해 마르텐사이트 격자로 변형됩니다. 마텐자이트 격자의 정방형 왜곡 정도 c/a는 마텐자이트의 C 농도에 따라 직접적으로 커집니다. 마르텐사이트 격자는 실온에서 정방형을 유지합니다. 초기 단계와 마르텐사이트 단계의 방향 관계가 설정되었습니다.
마르텐사이트 핵 생성의 특성에 대한 많은 가설이 있습니다. 그들 중 다수는 시작 매트릭스의 특수 결함 부위에서 이질적인 핵 생성을 옹호합니다.
마르텐사이트는 형태와 관련하여 두 가지 기본 유형으로 나뉩니다. 이들은 판 마르텐사이트와 거대 마르텐사이트입니다. 그들은 모양, 결정의 상호 배열, 하부 구조 및 습관 평면이 다릅니다. 판(바늘) 마르텐사이트는 높은 C 강철에서 더 자주 발견됩니다. 마르텐사이트 결정은 얇은 렌티큘러 플레이트 모양입니다. 처음으로 나타나는 판은 장치 전체를 통과하여 별도의 부분으로 나눕니다. 그러나 그들은 매트릭스 입자 경계를 넘을 수 없습니다. 따라서 플레이트 치수는 오스테나이트 입자의 치수에 의해 제한됩니다. 새로운 마르텐사이트 판은 오스테나이트 단면에 형성됩니다. 여기서 플레이트 치수는 단면 치수로 제한됩니다. 오스테나이트 결정립이 작으면 마르텐사이트 판은 너무 미세하여 미세단면 시편에서 마르텐사이트의 침 구조를 볼 수 없습니다. 이러한 마르텐사이트를 구조가 적은 마르텐사이트라고 하며 가장 바람직합니다.
저탄소강과 중탄소강에서 다량의 마르텐사이트를 관찰할 수 있습니다. 이러한 유형의 마텐자이트 결정은 거의 동일한 방향을 갖는 상호 연결된 판으로 형성됩니다. 거대한 마르텐사이트 판은 낮은 각도 경계로 분리됩니다.
베이나이트의 변형
베이나이트의 변태는 펄라이트 변태와 마르텐사이트 변태의 중간이다. 베이나이트의 변태 동역학과 형성된 조직은 확산 펄라이트 변태와 확산이 적은 마르텐사이트 변태의 특징을 모두 보여줍니다. 이 변형의 결과로 페라이트와 탄화물의 혼합물이 형성됩니다. 이 혼합물을 베이나이트라고 합니다. 베이나이트 변환 메커니즘은 격자의 감마에서 알파로의 재배열, C의 재분배 및 탄화물의 침전을 포함합니다.
펄라이트 및 마르텐사이트 대응물에 대한 베이나이트 변태의 근접성이 여기에서 설명됩니다. 베이나이트 변태 범위에서는 기본 성분인 Fe 원자의 확산 운동이 거의 완전히 억제됩니다. 그러면 펄라이트 석출의 억제로 인해 페라이트의 감마-알파 형성이 어렵습니다. 그러나 C 확산은 다소 활발하여 탄화물의 침전을 유발합니다. 중간 범위에서 감마상 결정은 마르텐사이트 판과 같이 응집성 성장을 통해 형성됩니다. 그러나 알파 위상판은 순간적으로 형성되지 않고 천천히 형성됩니다.
This is due to the fact that over the intermediate temperature range the alpha phase can precipitate only from the C depleted gamma phase. Thus the growth rate of the alpha phase crystals depends on the C diffusive removal rate. In this case, the martensite start point Ms in austenite rises and the martensite gamma to alpha transformation takes place at temperatures above the temperature Ms typical of the steel with a given composition.
At the instant of martensite transformation, the C concentration remains unchanged. Only the crystal lattice is altered and a supersaturated a solution is formed. Carbide precipitates after gamma to alpha transformation.
There is a difference between upper and lower bainite, which are formed in the upper and lower parts of the intermediate temperature range. The conventional boundary between the bainite is close to 350 deg C. Upper bainite has a feathery structure, whereas lower bainite shows an acicular morphology, which is close to that of martensite. The difference in the structures of upper and lower bainite is due to the difference in the mobility of C in the upper and lower parts of the bainite temperature range.
The alpha phase substructure of upper bainite resembles the substructure of massive martensite in low C steel, while the alpha phase structure of lower bainite approaches the structure of martensite in high C steels. In upper bainite, carbide particles can precipitate both at lath boundaries and inside laths. This fact suggests that here carbides precipitate directly from austenite. In lower bainite, carbide is found inside the alpha phase. This is since carbide is formed during precipitation of a supersaturated solid solution of C in the alpha phase. Both upper and lower bainite shows a high density of dislocations inside the alpha phase. Fe3C is the carbide phase in upper bainite and epsilon carbide (Fe2C) in lower bainite. As the holding time is increased, Fe2C turns into cementite. The dimensions of austenite grain have no effect on the kinetics of martensite transformation.
Tempering
The processes which take place during tempering are precipitation and recrystallization of martensite. Quenched steel has a metastable structure. If subjected to heating, the structure becomes closer to equilibrium. The nature of the processes which occur during tempering is determined by three major characteristics of quenched steel namely (i) strong super saturation of the martensite solid solution, (ii) high density of crystal lattice defects (dislocations, low angle and large angle boundaries, and twin interlayers etc.), and (iii) presence of retained austenite.
The main process taking place during tempering of steel is the precipitation of martensite accompanied by formation of carbides. Depending on the temperature and duration of tempering, the martensite precipitation can involve three stages namely (i) pre-precipitation, (ii) precipitation of intermediate metastable carbides, and (iii) precipitation and coagulation of cementite. Retained austenite can precipitate simultaneously. Since there is a high density of dislocations in martensite, hence its substructure is similar to the substructure of steel which is work hardened. Hence, polygonization and recrystallization can develop during tempering.
When C steel is tempered, super-saturation of the gamma solution in austenite increases with an increase in the C content of steel. This leads to lowering of the Ms-temperature and transition from massive martensite to plate martensite. The amount of retained austenite also increases.
The segregation of C represents the first structural changes which take place during tempering of C steel. The segregated C can nucleate heterogeneously at lattice defects or homogeneously in the matrix. The heterogeneous nucleation of the segregated C takes place either during quenching or immediately after it.
Flat homogeneous clusters of C atoms not connected with lattice defects are formed at tempering temperatures of less than 100 deg C. This is due to the considerable displacements of Fe atoms and the appearance of elastic distortions. As the tempering temperature is increased, the clusters become larger and their composition is close to Fe4C. This process is dependent on the C diffusion. Metastable Fe2C is formed above 100 deg C. It has a hexagonal lattice and appears directly from C clusters when the C concentration is increased. Metastable Fe2C can also precipitate directly from the alpha solution. Fe2C precipitates as very fine (10 nm to 100 nm) plates or rods at low temperatures. With an increase in tempering temperature or time, Fe2C particles become coarser and precipitate in steels containing a minimum of 0.2 % C. In steels with a high Ms-temperature, partial precipitation of martensite is associated by the deposition of excess carbide and is obtained during quench cooling in the martensite range. Hence self-tempering of these steels occurs during their quenching.
Cementite is formed at a temperature higher than 250 deg C. Two known mechanisms of Fe3C nucleation are (i) precipitation directly from a supersaturated alpha solid solution and growth of Fe3C particles at the expense of the dissolution of less stable carbides, and (ii) appearance of Fe3C as a result of transformation of the intermediate carbide lattice to the Fe3C lattice.
In the final stage of the carbide formation during tempering, coagulation and spheroidization of carbide take place. These happen intensively starting from 350 deg C to 400 deg C. At temperatures higher than 600 deg C, all Fe3C particles have a spherical shape and undergo coagulation only.
A substantial part of the tempering process is devoted to the precipitation of retained austenite accompanied by deposition of carbides. Precipitation occurs over the temperature range of 200 deg C to 300 deg C. During tempering, retained austenite transforms into lower bainite.
A decrease in the C concentration of the alpha phase during carbide formation results into changes in the phase structure. Martensite precipitation is conventionally divided into two stages. The first stage of precipitation is achieved below 150 deg C when the mobility of C atoms is sufficient for the formation of carbide plates. But, it is insufficient for the carbide plates to grow by diffusion of C from the areas of non-precipitated martensite with a high C concentration. This results in a non-uniform content of C in different areas of the martensite and hence inhomogeneity of martensite results with respect to its tetragonality. In areas with precipitated carbide, tetragonality is lower than in non-precipitated areas. Two solid solutions with different C concentrations coexist. For this reason the precipitation is referred to as a two -phase precipitation. The two phase precipitation of martensite results from the deposition of new carbide particles in areas containing martensite with the initial C concentration. Carbide particles do not grow at this stage.
At the second stage of martensite precipitation (150 deg C to300 deg C the alpha solution is depleted of C owing to diffusive growth of carbide particles, but the process proceeds very slowly. Hence, the precipitation kinetics is due to the rapid depletion of the alpha solution in carbon. Subsequently, depletion of the solid solution in C stops. At 300 deg C around 0.1 % C is left in the alpha solution. Above this temperature, no difference between the lattice of the alpha solution and that of the alpha-Fe is detected. Below 300 deg C the degree of tetragonality is still measurable. Above 400 deg C the alpha solution becomes completely free of excess C and transformation of martensite to ferrite is finished.
Plates (needles) of quench martensite have a high density of dislocations which is comparable to the density of the deformed steel. However, recrystallization centres and their progress to recrystallized grains are not observed. This is since carbide particles pin dislocations and large angle boundaries. It is only above 600 deg C, when the density of the particles decreases owing to the coagulation, that the recrystallization growth of grains takes place at the expense of migration of large angle boundaries. With this the morphological structures of lath martensite disappear. These processes are hampered in high C steels as compared to low C steels, since the density of carbides is greater in high C steels. The acicular structure is retained up to the tempering temperature of around 650 deg C.
The structural changes which occur during tempering cause alteration of steel properties. These changes depend on the tempering temperature and time. Hardness decreases as the tempering temperature is increased.
Kinetics of transformation of austenite
The kinetics of transformation of austenite is described below.
Isothermal transformation diagrams
It is important to follow the process at a constant temperature for the understanding of the kinetics of the transformation to austenite. For this purpose, isothermal transformation (IT) diagram is usually made which illustrates the isothermal process of austenite precipitation. In IT diagram (Fig 2), the transformation time is in the X-axis shown on the logarithmic scale and the temperature is plotted on the Y-axis. From this diagram, the incubation period (left hand curve) can be determined and also the time required for completion of the process (right hand curve). The instant, steel passes the points A3 and A1 during quenching, is usually taken as the zero time reference.
The time required to achieve the temperature of the quenching medium is frequently neglected. The start and finish of the transformation are difficult to determine from the transformation curve behaviour at the initial and final sections of the curve. Hence, the lines of the IT diagram generally correspond to a certain final volume which has undergone transformation, e.g., 2 % and 98 % for the transformation start and finish, respectively. The volume value is usually not shown in the IT diagram.
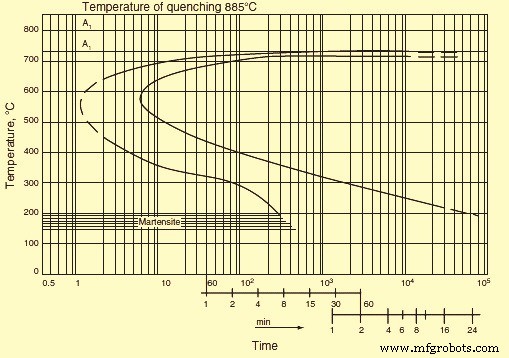
Fig 2 Isothermal transformation diagram
In addition to the curves stated above, IT diagram frequently contains intermediate curves corresponding to certain values of the transformed volume, say 20 %, 50 %, or 80 %. A decrease in the transformation rate causes displacement of the transformation start and finish curves to the right, i.e., toward greater duration. This phenomenon can be seen if the quenching heating temperature increases as a result of a decrease in the number of inclusions and growth of austenite grains. An increase in the transformation rate leads to displacement of the curves to the left. This phenomenon can be accounted for (i) by a decrease in the quenching heating temperature, (ii) the presence of carbides or inclusions, and (iii) refinement of the austenite grain. For a specified sample of steel the temperature which corresponds to a maxi mum transformation rate (the nose of the sigmoid curve) does not, as a rule, change significantly.
Continuous cooling transformation diagrams
Continuous cooling transformation (CCT) diagrams consider the transformation kinetics of eutectoid steel. The major transformation which takes place during annealing cooling of steel is a eutectoid precipitation of austenite into a mixture of ferrite and carbide. The eutectoid transformation kinetics is given by IT diagrams of austenite at a temperature of 727 deg C. The structure attained after tempering below 300 deg C is called tempered martensite. An acicular structure is seen after tempering at 300 deg C to 450 deg C. Tempering over the temperature interval of 450 deg C to 600 deg C shows a distinct dot structure. Austenite is in a thermodynamically stable equilibrium with the ferrite-Fe3C mixture. Stability of undercooled austenite is defined by a period of time during which the appearance of precipitation products in the diagram cannot be registered by conventional methods. The degree of austenite undercooling is the main factor which determines the steel microstructure. The necessary degree of undercooling is provided by either continuous cooling or isothermal treatment.
As seen earlier, in hypo-eutectoid steels the formation of pearlite is preceded by precipitation of hypo-eutectoid ferrite. With a decrease in the transformation temperature and an increase in the degree of undercooling, precipitation of hypo-eutectoid ferrite is suppressed. The amount of pearlite increases and the C content becomes less than that in pearlite of the eutectoid steel. In the region of the maximum transformation rate, the two curves merge. Thus, a purely pearlitic structure is formed in steel with 0.4 % C. In steels containing higher amounts of C, the precipitation of ferrite cannot be suppressed even if the C content decreases. Ferrite precipitation precedes the formation of pearlite even at a maximum transformation rate, but the amount of ferrite is less than that is formed at smaller undercooling.
These propositions are valid for the precipitation of cementite in hyper-eutectoid steels, but it can be suppressed even at relatively small undercooling. In this case, the C content of pearlite becomes higher than that in the eutectoid steel. As a result of suppression of the hypo-eutectoid ferrite precipitation under continuous cooling from the region of the gamma solid solution, the point Ar3 lowers much faster than the point Ar1 as the cooling rate is increased. With a certain cooling rate, both points merge into one point, which corresponds to the formation of a fine plate structure of the pearlite type free of ferrite.
Under continuous cooling the transformation process can also be visualized as diagram in temperature-time coordinates. Therefore the behaviour of cooling curves is to be analyzed to find characteristics of the transformation processes. In this diagram, the ferrite and pearlite start lines are shifted toward longer periods of time compared to the IT diagram. This is due to an increase in the temperature interval necessary for preparing the transformation processes in the austenite lattice. As a result, only part of the incubation period, which is needed for the IT to start, is effective. In this case, the incubation period is the mean of the effective lengths of time corresponding to different periods of time in the given range. This proposition can be used to calculate the behaviour of the transformation start line in the pearlite range from the IT diagram. The reverse calculation is also possible.
Similar to the pearlite range, in the bainite temperature range, the precipitation of undercooled austenite starts after a certain incubation period. Resemblance of the bainite and pearlite transformation kinetics consists not only in the presence of an incubation period but also in the character of the volume increase during isothermal soaking which is the fraction of the transformed volume of austenite increases first with acceleration and then with deceleration. At the same time, as in the case of the martensite transformation, retained austenite does not disappear completely during the bainite transformation. Every point in the bainite finish curve corresponds to certain amount of retained austenite. Similar to the pearlite transformation, the bainite transformation can take place both during isothermal soaking and under continuous cooling. Austenite which has not been transformed over the bainite range turns partially into martensite when the steel is cooled to room temperature. Since the austenite is inhomogeneous with respect to the C content after the bainite transformation, martensite is formed predominantly in C enriched regions.
For the high alloy steel, IT curves can be separated by a temperature interval in which undercooled austenite is highly stable. In this interval, pearlite precipitation does not take place for many hours, while undercooling is inadequate for the bainite transformation. In C steel, the bainite transformation proceeds concurrently with the pearlite transformation. Products of the pearlite transformation dominate at higher temperatures, and those of the bainite transformation at lower temperatures.
During the transformations of austenite on cooling in the martensite range, martensite component in the steel structure appears when the cooling rate achieves a certain value. The minimum cooling rate at which the martensite component is formed is called the lower critical rate of cooling. The rate at which transformations by the pearlite and bainite mechanisms are suppressed completely is referred to as the upper critical rate of cooling (quenching). If the conditions of austenite formation (austenitization temperature and the holding time at this temperature) and the cooling conditions (cooling rate exceeds the upper critical rate) are constant, the location of the martensite start point Ms depends only on the contents of C and alloying elements in the steel.
If the cooling rate is high, the formation rate of separate needles of martensite is also high, and transformation of austenite to martensite begins on reaching Ms-temperature. It continues on subsequent cooling to lower temperatures. As the temperature of the quenching medium is lowered, the amount of formed martensite increases first quickly and then slowly. With an increase in the quenching heating temperature (austenitization temperature), the transformation also shifts toward lower temperatures as more of the alloying elements are taken into solution. A certain amount of martensite can be formed during isothermal holding, but it is not high in C steels. Retained austenite is stabilized during isothermal holding. As a result, more martensite is formed during subsequent cooling. Formation of martensite stops at the point Mf. There is a relationship between some factors which influence the stabilization of martensite. The effect of stabilization increases with the amount of martensite in the structure or, the amount of martensite being equal, with temperature.
There is a close link between the CCT and IT diagrams. When resolving practical issues involved in heat treatment of steel, it is sometimes necessary to know how the continuous cooling rate affects the structure formed as a result of austenite transformation. For this, there have been efforts to establish the relationship between the transformation kinetics of austenite under isothermal conditions and under continuous cooling conditions. The efforts have started from the concept of additivity of the transformation processes at different temperatures. It has been presumed that holding of undercooled austenite at a preset temperature is part of the incubation period. However, it has been found, that calculated and experimental data coincide satisfactorily only if the pearlite transformation is continuous.
If the pearlite transformation is preceded by precipitation of eutectoid pearlite or the pearlite and bainite transformations occur concurrently, calculated data are at a discrepancy with the experimental data. It has been found that the discrepancy is due to the factors namely (i) holding of austenite during the time accounting for fractions of the incubation period causes acceleration of the subsequent intermediate transformation at the expense of preparatory processes, (ii) precipitation of hypo-eutectoid ferrite alters the austenite composition which delays the subsequent intermediate transformation, (iii) partial transformation of austenite over the intermediate range reduces the rate of the said trans formation at lower temperatures and facilitates an increase in retained austenite which is due to a redistribution of C and enrichment of the non-transformed part of austenite in carbon, and (iv) a change in the cooling rate over the martensite range affects stabilization of austenite in different ways.
For the above reason, special methods of constructing thermo-kinetic transformation diagrams of austenite subject to continuous cooling have been elaborated for non-eutectoid steels. From these diagrams it is possible to determine the critical rate of quenching cooling or continuous cooling which is necessary to complete a particular stage of austenite precipitation.
It has been seen that the CCT diagram is a function of the bar diameter. When steel is subjected to martensitic hardening, it is required to be cooled from the quenching temperature so that on undercooling to a temperature below the Ms point austenite has no time to precipitate and form a ferrite-carbide mixture. For achieving this, the cooling rate is to be less than the critical value. The critical cooling rate is the minimum rate at which austenite does not precipitate to a ferrite-carbide mixture. Of course, the cooling rate of steel products is non-uniform over their cross section. It can be higher than the critical rate on the surface and lower than the critical rate at the centre.
The critical cooling rate at different points of a product can be directly determined from an IT diagram. In the first approximation, it is given by the slope of the tangent to the C curve which denotes the austenite precipitation onset. This method gives a value which is around 1.5 times the true critical rate. The cooling rate can be determined more accurately if thermo-kinetic diagrams are used. Intercepts of the cooling curves with the lines of the thermo-kinetic diagrams show the start and finish temperatures of the corresponding transformation.
From the transformation diagram, it is possible to determine, for example, the rate which provides 40 % martensite in the structure or the rates at which the entire transformation occurs in the pearlite range, i.e., hardening is omitted altogether. Because the data on the critical hardening rate depend on cooling time and is to be associated with a specific temperature (at which direct measurements of the hardening rate are practically impossible), it is proper to specify the cooling time for a specific interval of temperature, for example, from the point A3 to 500 deg C. Point A3 in the diagram is the time reference. Then it is possible to directly determine the critical cooling time K (Km for fully martensitic hardening, Kf for initial appearance of ferrite, and Kp for full transformation in the pearlite range).
Since the cooling time and the progress of the subsequent cooling of the sample during end-face hardening are known, the outcome of hardening can be determined from the transformation diagram. It is to be remembered that a transformation diagram is valid only for particular conditions of melting and homogenization. Deviations in the composition or grain dimensions cause changes in the trend of thermodynamic curves. This is explained by the fact that an increase in the homogenization temperature and time and, consequently, enlargement of the grains enhance the stability of austenite. Conversely, refinement of grains lowers the critical cooling rate, since stability of austenite decreases with an increase in the extent of grain boundaries.
Hardenability
The depth of the hardened zone is termed hardenability. This is one of the most important characteristics of steel. Since the cooling rate is non-uniform along the cross section of a sample, austenite can pass into martensite in surface layers only, while at the centre of the sample austenite undergoes the pearlite transformation. In the first place, hardenability depends on the critical cooling rate. An examination of the temperature curves plotted for different areas of the sample shows that the cooling rate of the core of a large diameter product is lower than the critical value and hence the core is not martensitically hardened. Martensite is present in the surface layer only.
After hardening treatment, a bulky part with a large cross section can show the entire range of structures such as a smooth transition from martensite near the surface through troostite-martensite and troostite to pearlite at the centre. The geometry of samples can influence the character of the cooling curves. However, given the same surface-to-volume ratio, the curves coincide in general. The highest changes in the cooling rate are experienced by the diameter of samples.
Considering the above, for achieving a through hardening of bulky products or full martensitic hardening to the core of a product, it is essential to provide the critical hardening rate along the entire cross section of the product. IT and CCT diagrams can be used to determine this rate. The diagrams are usually plotted for different grades of steel, taking into account the progress of cooling in different sections and in different hardening media.
The hardenability of steels depends on the steel composition, specifically on the C content. In the steel hardenability diagrams, the hardenability of each grade of steel is normally presented as a hardenability band. These diagrams have been plotted for almost all existing grades of steel. They show how to achieve hardening of a product made of particular steel.
Hardenability of steel is also categorized by IT curves. The more the curve is shifted to the right along the X-axis, the greater is the hardenability of the steel. This is explained by the fact that the rightward shift of the IT curve is due to better stability of austenite.
An improvement in the stability of undercooled austenite and hence an increase in the critical hardening rate lead to a greater depth of hardening. Then hardenability depends on all the factors which improve the stability of undercooled austenite. As an example, the stability of austenite can be raised by alloying steel with chromium and tungsten. These elements lower the austenite precipitation rate and can make steel an air-hardening one. Steel with a normal content of impurities is hardened to strength ten times that of a pure Fe-C alloy.
Elevation of the hardening temperature favours an increase in the hardening depth due to the homogenization of austenite and enlargement of austenite grains. Refinement of grains impairs hardenability as grain boundaries affect the stability of austenite. The hardening depth also depends on the hardening medium used. The greater is the intensity of cooling, the greater is the depth of hardening. Besides, the hardening depth depends on the cross-sectional diameter of the products. The critical diameter is that of the greatest cross section which lends itself to through hardening in a given hardening medium. The critical diameter is different for different hardening media and characterizes the hardenability provided by a particular method only.
Hardenability has an effect on the mechanical properties of steel. In the case of through hardening, the properties do not differ along the cross section of a product. Otherwise they decrease from the surface to the centre. The analysis of the influence of hardenability on the properties of steels which have been tempered after hardening shows that a high temperature favours equalization of hardness along the cross section. However, the structure of weakly hardenable steels remains inhomogeneous. This is due to a grain structure appearing on the surface, where martensite is formed during quenching, while a lamellar structure remains at the centre. A grain structure is present along the entire cross section of through-hardening steel. This determines the character of changes in the properties of steels with different hardenability. The properties which are independent of the Fe3C form (YS, specific elongation, impact strength) differ.
The properties of tempered steels (fracture stress, YS, impact strength, reduction in area) are impaired if ferrite precipitates during quenching. The mechanical properties of a product depend on its cross-sectional area. To obtain the best mechanical properties in the tempered state, a grain structure is required to be provided along the entire cross section; i.e., through hardenability is to be ensured in the quenched state.
Grain size
It is necessary to know the material structure while analyzing any processes or properties associated with grain boundaries. Most of the steel materials have polycrystalline structure and they comprise a set of grains separated by boundaries. The grain boundary is one of the basic structural elements in polycrystalline steel materials. The grain boundary represents an interface between two differently oriented crystals. This is the region of crystal imperfection. It is capable of moving and adsorbing impurities. The boundary has a high diffusive permeability.
In polycrystalline steel materials, the boundaries determine the kinetics of many processes. For example, movement of grain boundaries controls the process of recrystallization. A high diffusive permeability of grain boundaries determines the kinetics of diffusion-dependent processes at moderate temperatures. Embrittlement of steel material is connected with enrichment of grain boundaries in impurities.
Grain boundaries are normally divided into two large groups namely (i) low angle boundaries, and (ii) large angle boundaries. Low angle boundaries are sub-grain boundaries with an angle of less than 10 degrees. They represent networks or walls of dislocations. The structure of large angle boundaries is much more complicated. The progress in understanding the structure of grain boundaries is connected with elaboration of the models describing the observed microscopic properties of the boundaries.
Grain size determination
The size of the grain that is formed under a given treatment is determined from micro-sections after their etching. For C and alloyed steels the reagent used is 1ml to 5 ml HNO3 +100 ml ethyl or methyl alcohol. Austenitic steel is etched in a copper sulphate-chloride solution containing 10 grams copper sulphate, 50 ml hydrochloric acid, and 50 ml water. When C and low alloy steels are etched, the reagents turn pearlite dark and make visible the ferrite grain boundaries, the martensite structure, and tempering products. The etching rate rises with the amount of nitric acid. The etching time is from several seconds to a minute. Etching of austenitic steel reveals the austenite structure and the austenite grain boundaries.
Carburization is also used to establish the austenite grain boundaries. In this case, samples are heated to 930 deg C in a carburizing medium (e.g., a mixture of 40 % BaCO3 and 60 % charcoal), cooled, and etched.
In addition, an oxidation method is used according to which micro-sections are heated in vacuum to a temperature 20 deg C to 30 deg C higher than the quenching temperature and are soaked for 3 hours. Subsequently air is fed to the furnace for 30seconds to 60 seconds, and the samples are cooled in water. Before quenching it is desired to heat samples in borax melt at 930 deg C to 950 deg C for 30 seconds to 40 seconds and then cool them in water. After these treatments micro-sections are polished and etched in a 15 % solution of hydrochloric acid in ethyl alcohol. Grain boundaries are seen as the oxide network.
Apart from this, use is made of the method of etching austenite grain boundaries, the method of the network of ferrite (for steels with a C content of up to 0.6 %) or Fe3C (for hypereutectoid steels), and the method of the pearlite network for steels which are closer in composition to eutectoid steels.
The grain size is determined by comparing the observed microstructure at a 100x magnification with standard scales (the scales are elaborated so that at a magnification of 100x the grain number N corresponds to the formula ‘n =8 X 2 to the power n’, with n the number of grains per sq mm of the micro-section area) or by counting the number of grains per unit area of the micro-section, or by calculating the mean nominal diameter of the grains or their number per cubic millimeter.
The austenite grain boundary structure which is produced on heating above the critical points is important since the austenite transformation products formed during cooling (martensite and pearlite etc.) appear inside austenite crystals. A coarse austenite grain determines a coarse plate structure of martensite during quenching or a coarse cellular network of ferrite (cementite) precipitates at the boundary of the initial austenite grains during annealing or normalization. The pearlite structure is also the coarser and the larger is the pearlite grain.
As is known, a coarse grain structure of steel (ferrite-pearlite, martensite, etc.) is characterized by lower mechanical properties. For this reason a fine-grain structure of steel is desirable in practice.
Grain size refinement
It is possible to refine a coarse-grained structure and this is widely used in the heat treatment of steel. The grain refinement, which takes place on heating steels above the Ac3 temperature, is related to a transition to the austenite state through nucleation of numerous centres of the austenite phase. Development of these centres leads to formation of a relatively fine grained structure. Above Ac3 temperature, the cross sectional size of the grain is 10 mm -30 mm. Initially the grain size is independent of the grain of the starting structure. It can be very fine irrespective of whether the starting structure of the steel is fine or coarse. A fine grain structure of the restored austenite provides a fine grain structure of cooled steel irrespective of the structural components (pearlite, bainite, or martensite) which are formed. This is due to the fact that all the transformation products nucleate within each separate grain of austenite.
Excess phases (ferrite in hypo-eutectoid steel and Fe3C in hyper-eutectoid steel) precipitate at boundaries of small austenite grains, and the pearlite transformation is accompanied by the appearance of smaller pearlite colonies. Fine austenite grains determine the formation of fine-needle martensite. This underlies the grain refinement effect which is associated with heating above Ac3 temperature. Heating the steel above Ac3 temperature during full annealing, normalization, or quenching is followed by recrystallization. With an initially coarse grain structure, recrystallization results in refinement of grains at a heating temperature corresponding to Ac3 temperature.
If the heating temperature is much higher than Ac3 temperature, then the grain is enlarged again, and the expected correction of the structure during the gamma to alpha transformation does not take place. Refinement of crystallites is especially pronounced when transformation to the austenite state starts in many centres inside the initial structure. The formed centres are to have a random orientation, which is not connected with the orientation of the alpha phase in the initial structure. Normally such centres are sufficiently large in number so that the grain size does not exceed 15 mm to 30 mm. During pearlite precipitation of austenite, breaking of an austenite grain into pearlite colonies, each of which can be considered an independent grain, also represents refinement of steel.
Strengthening mechanism in steel
There are four strengthening mechanisms in steel namely (i) solid solution strengthening, (ii) grain size refinement, (iii) dispersion strengthening, and (iv) work hardening.
Solid solution strengthening is a phenomenon which occurs when the number of impurity atoms in the lattice of the basic element is so small that they are incapable of forming both stable and metastable precipitation phases under any heat treatment conditions. However the impurity atoms favour improvement of the mechanical properties. The presence of impurity atoms in the matrix lattice leads to distortion of the lattice because of the difference in size between the atomic radii of the impurity and the basic component. This in turn leads to the appearance of elastic deformation fields, which retard movement of dislocations in slip planes under the action of applied stresses. In addition, the impurity atoms can obstruct movement of dislocations by forming impurity atmospheres around them. Both of the above factors play a leading role in solid solution strengthening.
Carbon which is statistically uniformly distributed in the lattice of the alpha iron has an influence on the structure and properties of alpha iron. Solubility of C in alpha iron is much lower than in the gamma iron. It forms interstitial solid solutions with both types of irons. However, whereas the gamma iron lattice has sufficiently large pores for implantation of C atoms, the cubic lattice of the alpha iron suffers. Upon introduction of C atoms, a tetragonal distortion takes place which is similar to the one of the martensite lattice except that in the former case the distortion is much smaller. In addition, inserting of C atoms causes the entire lattice of the alpha iron to somewhat expand. Hence, C affects the properties of the alpha phase. Actually, there is a dependence of the YS on the C concentration in the solid alpha solution. The influence which C exerts on plastic deformation resistance of the alpha phase is due to its strong interaction with dislocations as well as pinning of the dislocations and elastic deformations arising as a result of the tetragonal distortion of the alpha phase lattice after insertion of C atoms.
The presence of C in lattices of different structural components formed during thermal treatment of steel also leads to changes in their mechanical properties. As an example, the location of inserted C atoms primarily in one of the sub-lattices of interstitial sites during the martensite formation brings about additional tetragonal distortions of the martensite crystal lattice. This enhances plastic deformation resistance owing to the interaction between the stress fields around C atoms and those at dislocations. The influence of C dissolved in the alpha phase on the mechanical properties of steel is also witnessed in the case of the ferrite – pearlite transformation. The dissolution of part of the C in the alpha phase suggests that the solid solution strengthening of the phase is one of the factors providing the high strength properties of intermediate transformation products.
Grain size refinement of steel has a strengthening effect on steel. Impact strength is especially sensitive to the austenite grain size, and it decreases with grain enlargement. A decrease in the dimensions of pearlite colonies inside the initial austenite grain also favours a rise in impact strength.
Although the grain size has a considerable effect on impact strength, its influence is small if any on the individual mechanical properties such as hardness, fracture stress, YS, and specific elongation. Only the actual grain size affects steel properties, the inherited size has no effect. However, the technological process of heat treatment is determined by the inherited grain.
In the steels, precipitation of supersaturated solid solutions formed during quenching is followed by precipitation of disperse particles enriched in atoms of the alloying components. The strength (hardness) of the steels increases with the precipitation of these particles. The increment in the value of these characteristics increases as the dispersion and volume fraction of the particles increase. This phenomenon has been referred to as dispersion strengthening.
Precipitation of supersaturated solid solutions takes place during the heating (aging) of quenched steels. The strengthening is due to an increase in resistance to the movement of dislocations in a crystal when obstacles (barriers) of any type are formed. In aging steels, dislocations meet regions which retard their movement. The character of interaction between moving dislocations and precipitates of the second phase can be different depending on the phase morphology and structure. The total effect of aging on the strength properties of steels is determined by (i) the strength of the precipitates formed, (ii) the volume fraction of precipitates, (iii) the degree of precipitate dispersion, (iv) morphology, structure, and type of binding with the matrix, and (v) temperature.
When a solid solution of C in alpha Fe is cooled below A1 temperature, C precipitates as Fe3C with lowering of the C solubility and a decrease in temperature. This process takes place under sufficiently slow cooling, which is accompanied by diffusion processes, leading to the formation of cementite. In the case of abrupt cooling (water quenching) C has no time to precipitate. A super-saturated alpha solid solution appears. During subsequent storage at room temperature (natural aging) C tends to precipitate from the solid solution. Carbon enriched regions appear primarily in defective sections of the matrix. Precipitation of C from a supersaturated solid solution during natural aging results in an improvement of its strength properties and hardness. However, plastic properties such as reduction in area, specific elongation, and impact strength are deteriorated and the phenomenon of dispersion strengthening is seen.
As the heating temperature is increased (artificial aging), dispersion strengthening accelerates. This is due to the intensification of diffusion processes with an increase in temperature. The total process of C precipitation from the super-saturated solid solution in alpha Fe comprises several successive processes. Mechanical properties and hardness are not sensitive to structural changes which take place during the aging of the steels. Sharp changes in properties indicate alterations in the structural state of the steel.
A maximum change in mechanical properties during precipitation is achieved only if excess crystals in a highly disperse state precipitate. Subsequent coagulation of the crystals leads to degradation of the properties.
The influence of different solubilities of C in alpha Fe on the properties of the steel (dispersion strengthening) during low temperature aging is prominent in low C steels. In steels containing C higher than 0.4 %, the above effects are not noticed due to the influence of Fe3C particles formed during the pearlite transformation. Besides, nucleation of the precipitating phase can be inhibited owing to migration of C to the Fe3C-ferrite interfaces. As a result, the amount of C concentration at lattice defects decreases.
Cold plastic deformation greatly accelerates precipitation of a supersaturated solid solution. This is due to an increase in the density of dislocations, which are preferable sites of heterogeneous nucleation of precipitates as well as to an increase in the concentration of vacancies, which facilitates the diffusion of C to clusters. Mechanical properties change during aging after cold working in the same way as after quenching, that is, the YS, the fracture stress, and hardness are altered. With an increase in aging time, specific elongation and reduction in area decrease and the tendency to brittle fracture is enhanced. The rate of change is higher than in quenched steel. Also, the nature of the changes is different. Whereas in the case of aging after quenching, hardness reaches a maximum and then drops, after cold working hardness does not decrease with the aging time. As the aging temperature is raised, the maximum hardness of quenched steel lowers, while after cold working hardness is independent of the aging temperature. This is explained by the fact that a considerable amount of C is concentrated near dislocations. Few, if any, clusters nucleate in the matrix homogeneously. Consequently, clusters cannot grow at the expense of other clusters, i.e., they cannot coagulate.
An important method used to strengthen steels is deformation strengthening. Strengthening achieved with crystal deformation can be judged from the shape of stress-strain curves. The actual shape of these curves largely depends on the crystal lattice type of the metal, its purity, and thermal treatment.
In the case of cubic lattice steels, strengthening curves are parabolic, whereas for hexagonal lattice metals a nearly linear dependence is observed between the stress and the strain. This fact suggests that plastic deformation strengthening is determined mainly by the interaction of dislocations and is associated with the structural changes which retard the movement of dislocations. Metals with a hexagonal lattice are less prone to deformation strengthening than cubic lattice steels because the hexagonal lattice has fewer easy slip systems. In cubic lattice steels, the slip proceeds in several intersecting planes and directions.
There are three stages during the work hardening. The first stage is due to the easy slip. It depends on the orientation of the crystal relative to external forces and on the presence of impurities. This stage is characterized by a linear dependence of strain stresses on the strain at a small work hardening rate. Dislocations usually slip in primary systems.
In the second stage the work hardening rate is much higher than the first stage. Dislocations move in intersecting slip planes and, on colliding, form additional obstacles to their movement. This state is most extensive in the stress-strain curve. The ratio between the work hardening rate and the shear modulus (or any other elastic constant) is almost independent of the applied stress and temperature. It depends little on the crystal orientation and presence of impurities.
In the third stage changes are possible in the distribution of dislocations. They can either get around obstacles which retard their movement at the second stage or interact with dislocations. As a result, the work hardening rate is lower compared to which is observed during the second stage. At this stage, a partial relaxation of stresses can occur owing to the appearance of the secondary slip system. The reduction of distortion can have the result that deformation continues in the primary system, which gets rid of a certain number of dislocations passing to the system. A characteristic feature of deformation in the third stage is the development of a cross-slip representing the main mechanism by which dislocations bypass the obstacles formed in the second stage.
Heat treatment processes for steels
There are three basic processes for the heat treatment of steels. These are (i) annealing, (ii) quenching, and (iii) tempering.
Annealing
Annealing process of steels has different methods namely (i) diffusion annealing, (ii) softening, (iii) phase recrystallization annealing or full annealing (normalization, high temperature or coarse grain annealing, and pearlitization), and (iv) stress relief annealing and recrystallization annealing.
The objective of diffusion annealing is to eliminate, as far as possible, in-homogeneities in the chemical composition, in particular liquation in-homogeneities, which occur during crystallization of steels. This annealing is usually carried out in the range of the gamma solid solution at a temperature of 1100 deg C to 1300 deg C. Diffusion annealing can be used primarily to smoothen out a difference in the content of alloying elements, the difference being due to the inter-crystal liquation. This shows up as smearing of dendrites with an increase in temperature and heating time. Differences in micro-hardness are removed simultaneously. The overall hardness of the steel decreases since liquation regions possessing high hardness is removed. Some average hardness is attained. The success of diffusion annealing largely depends on the steel purity and liquation. This type of annealing is generally used to improve properties of medium purity steels.
Softening is used to produce the structure of globular pearlite. This structure is very soft and readily lends itself to deformation during drawing and cold rolling etc. Steels with a low C content become too soft after this annealing treatment. The globular pearlite structure is favourable in steels with a C concentration of more than 0.5 %. Another goal of softening is to produce a uniform fine structure with finely dispersed C after quenching. The simplest method of softening consists in holding for many hours at a temperature slightly above Ac1 temperature. In this case, martensite which is left from the previous treatment is removed and the work hardening caused by cold working is eliminated. Cooling after softening can be done in air starting from 600 deg C. Refinement of the structure subjected to softening is achieved only above the point A1 temperature.
Phase recrystallization annealing consists of a twofold gamma to alpha transformation, which takes place during this annealing. It leads to the appearance of a fine grained uniform structure differing completely from the initial structure. Refinement of the grain during normalization results in the disappearance of the Widmanstätten and coarse grained cast structures, which have poor mechanical properties. Inhomogeneity of the structure in the work hardened state is removed. The closer the annealing temperature is to Ac3 temperature and the shorter the holding time at this temperature, the finer is the grain. Refinement of the grain structure is also facilitated if the heating rate to the annealing temperature and the cooling rate from this temperature are increased.
In the case of normalization, cooling is done in air. Here it is important to allow for different rates of cooling along the cross section of large sized products. The arising thermal stresses are removed by stress relief annealing or high temperature tempering. To obtain a fine grained structure, rapid cooling is done only over the transformation temperature interval. The normalization heating temperature is not to be much higher than the transformation point, or else the grain may be too coarse (overheating). An excessively long holding time also have the same result. The optimal heating temperature is determined by the C content.
Stress relief annealing and recrystallization annealing removes macroscopic stresses which are present in cold worked steels due to the dislocation pile ups and crystal lattice distortions. Normally these stresses are very high. Changes in properties which occur during the cold working can be rectified during subsequent heating. The greater is the degree of cold working, the lower is the heating temperature. Depending on the temperature and time of annealing, various structural changes take place in a cold worked material. The changes are divided into recovery and recrystallization processes.
Recovery is a totality of any spontaneous process of variation in the density and distribution of defects before the onset of recrystallization. If recovery proceeds without the formation and migration of sub-grain boundaries inside the recrystallized grains, it is called restoring. If sub-grain boundaries are formed and migrate inside the crystallites, recovery is referred to as polygonization.
Restoring does not include an incubation period. Properties start changing right at the beginning of annealing. Restoring is accompanied by a redistribution of point defects whose concentration decreases subsequently from excess concentration to the equilibrium concentration. Simultaneously, dislocations are redistributed and unlike-sign dislocations are annihilated.
The structure of steel starts changing drastically from a certain annealing temperature. New rather equilibrium grains are seen along with extended cold worked grains. They differ from the grains of the deformed matrix by having a more perfect internal structure. As distinct from the polygonized structure, recrystallized grains are separated from the matrix with large angle boundaries.
The formation and growth of grains with a more perfect structure which are surrounded by large angle boundaries at the expense of initially cold worked grains of the same phase is called primary recrystallization. Recrystallization begins with an incubation period. The recrystallization rate increases initially from zero to a maximum and then decreases due to an ever rising number of new grains in contact with one another.
Inclusions of insoluble impurities (carbides, nitrides) lower the tendency to growth of recrystallized grains. This is especially important in the case of ferritic steels, which are prone to grain growth. Another phase can precipitate during recrystallization in alloy steels which are subjected to a strong cold working.
Sometimes the intensive growth of individual crystals can be seen after a strong deformation and long holding (for several days) at temperatures close to the melting point. This phenomenon is called secondary or collective recrystallization.
Quenching
Quenching is the strengthening treatment. It consists of cooling from the temperature range of the solid solution at such a rate that transformation in the primary and bainite ranges are suppressed and martensite is formed. In this state, steel has the property of the highest hardness. There is a distinction between (i) normal quenching, which is used mainly for treatment of medium C and high C steels, and (ii) quenching after a thermo-chemical treatment (carburization, high temperature cyaniding etc.), which is used for low C steels.
In case of normal quenching for providing a required cooling rate during quenching, different cooling media and methods are used. Water, oil, or air can serve as the cooling medium. Many alloy steels, which have a high stability of austenite, are subjected to step quenching. With this method of quenching, the temperature drop is less than in the case of direct cooling to room temperature and consequently quenching stresses are less.
Some quantity of austenite is retained during quenching even in steels with a relatively small C content. For this reason it is impossible to impart the maximum hardness to a product. Since austenite is stable at room temperature and passes to martensite at lower temperatures, steels are being given a subzero treatment. Under this treatment quenching is continued and steels with a high content of retained austenite are immersed in liquid nitrogen (N2) or quenching mixtures whose temperature is below 0 deg C.
For surface quenching (if it is necessary to harden only the surface layer to a preset depth), special quenching heating schedules are used. The surface of the steel is fully heated, while the core is cold and remains unquenched on subsequent fast cooling. The selection of steel for surface quenching is governed by the sensitivity of the steel to quick heating and cooling. For this reason the C concentration is limited to 0.7 % to prevent cracking of the steel.
Among the quenching defects, the main defects are excessive holding and overheating. They show up as enlargement of martensite needles and coarse grain fracture. This leads to a high brittleness of quenched steel and the formation of cracks. Cracks are frequently formed at the boundaries of initial austenite grains. A low quenching temperature or too short a holding time at the given temperature causes incomplete quenching. In this case, the quenched steel is insufficiently hard.
Carburization is the thermo-chemical treatment which is associated with surface saturation of steel with C and N2. These elements quickly dissolve in Fe by the interstitial method and are capable of rapid diffusion to a considerable depth. Low C steels are subject to carburization. Carburization is usually carried out at 900 deg C to 950 deg C. Gas carburization is used mostly, under which steel is heated in the atmosphere generated from a gas which contains predominantly methane (CH4) or from liquid hydrocarbons (kerosene and gasoline etc.). Carburization is aimed at enrichment of the surface layer with carbon. The required strengthening of the surface layer is achieved by quenching, which is done after the carburization. The specific volume of the quenched carburized layer is higher than the specific volume of the core, and hence considerable compression stresses arise in the layer. This improves the fatigue strength of the steels.
Cyaniding consists of the saturation of the surface of steels with C and N2 in a cyanide-containing salt bath. The C-N2 ratio in the diffusion layer is controlled by changing the medium’s composition and the processing temperature. Advantages of cyaniding over carburization consist in a shorter processing time and improvement in the wear and corrosion resistance (owing to the presence of N2 in the surface layer).
Tempering
The main purpose of tempering is to provide a disperse structure at a preset degree of cooling. In the case of low C steels, quenching serves as tempering even if the steel is not subjected to high temperature tempering it has a high viscosity and a relatively high strength.
When some of the steels are quenched in oil, a structure is formed even during transformation in the bainite range which is more disperse than the one formed after cooling in air. But the most disperse distribution of carbides and the most favorable properties are obtained after martensite tempering. The structure dispersion has the highest effect on the YS. An improvement of the fracture stress and YS and an increase in the fracture stress -YS ratio can be taken as a measure of the tempering efficiency. The tempering efficiency depends on the cross-sectional area and on the content of C and alloying elements in the steels.
Although to achieve a thorough quenching the critical quenching rate has to be exceeded over the entire cross section, full tempering does not require this procedure. As an example, in quenched steel which has martensite in the surface zone and pearlite in the core, the hardness of the core can sometimes be higher than that of the surface zone after tempering. This is particularly the case during a short tempering when precipitation of carbides from martensite proceeds faster than the coagulation of pearlite plates.
Tempering of hypo-eutectoid steels which do not contain free ferrite gives a uniform improved structure. In the presence of ferrite precipitates, the fracture stress-YS ratio decreases and the impact strength is lesser than in the surface zone. Hence, in selecting the content of C and alloying elements and particular conditions of austenitization and cooling, the size of the steel product to be tempered is required to be considered. For tempering to give adequate properties, it is often enough to suppress the formation of ferrite during continuous cooling. Only when a very high fracture stress is required an abrupt cooling is used for tempering. In this case, susceptibility to full tempering can be improved by raising the quenching temperature and thus enlarging the austenitic grain size.
제조공정
열처리 및 표면 처리와 같이 CNC 정밀 가공에서 일반적으로 사용되는 후속 공정이 많이 있습니다. 이러한 가공 기술은 정밀 부품을 보다 실용적으로 만들기 위한 것입니다. 열처리에는 가열, 보온, 냉각의 3단계가 있습니다. 열처리에서 중요한 역할을 하는 요소는 온도와 시간입니다. 열처리란 고체강을 일정한 온도로 가열하여 필요한 보온을 하고, 적당한 속도로 상온까지 냉각시켜 강재의 내부구조를 변화시켜 원하는 성능을 얻는 것이다. CNC 정밀 가공에서 열처리는 기계 부품의 기계적 기능을 개선하고 가공 품질이 상품 사용의 요구를 충족
SKD11 다이스틸은 일본의 공구강입니다. 재료 열처리 경도:hrc58-60 SKD11은 일종의 고탄소 및 고크롬 합금 공구강입니다. 열처리 후 높은 경도, 연삭성, 강한 경화성 및 우수한 치수 안정성을 갖습니다. 좋은 기계 가공성, 미세하고 균일한 탄화물 입자, 화학 원소인 몰리브덴과 바나듐의 특수 첨가로 인해 담금질 균열에 대해 걱정할 필요가 없습니다. SKD11 특정 성능 A) 고온 강도 및 인성, 우수한 내마모성, 쉬운 절단; B) 강도, 인성 및 내열성 균형이 우수한 냉간 가공 다이 강, 다) 진공 탈기 정제를